We have provided you with Extra and Important Questions of Class 10 Science Chapter 1 Chemical Reactions and Equations. This Extra and Important Questions will help you to score 100% in your Board Exams. These extra questions will be helpful to revise the important topics and concepts.
Join our Telegram Channel, there you will get various e-books for CBSE 2024 Boards exams for Class 9th, 10th, 11th, and 12th.
Chemical Reactions and Equations Class 10 Important Questions with Answers Science Chapter 1
Question: Distinguish between a displacement reaction and a double displacement reaction.
Answer: A displacement reaction is a reaction in which more reactive metal can displace less reactive metal from its salt solution.
Double displacement reactions are those reactions in which compounds exchange their ions to form two new compounds.
Question: When you have mixed the solutions of lead(II) nitrate and potassium iodide,
(i) what was the colour of the precipitate formed and can you name the precipitate?
(ii) write the balanced chemical equation for this reaction.
(iii) is this also a double displacement reaction?
Answer: (i) The colour of the precipitate is yellow. The name of compound formed as a precipitate is Pbl2 (lead iodide).

Question: What do you mean by exothermic and endothermic reactions? Give examples.
Answer: Exothermic reactions are those in which heat is evolved, e.g.
C(s) + O2(g) → CO2 + heat
CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) + heat
Endothermic reactions are those reactions in which heat is absorbed, e.g.
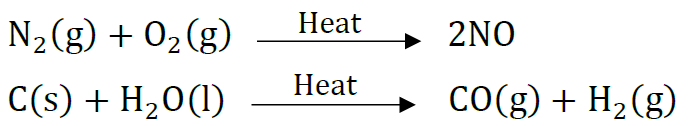
Question: Write the chemical equation of the reaction in which the following changes have taken place with an example of each:
(i) Change in colour
(ii) Change in temperature
(iii) Formation of a precipitate
Answer: (i) Cu(s) + 2AgNO3 (aq) → Cu(NO3)2(aq) + 2Ag
The solution will become blue in colour and shiny silver metal will be deposited.
(ii) NaOH + HCl → NaCl + H2O+ heat
The temperature will increase because heat will be evolved.
(iii) Pb(NO3)2 (aq) + 2KI (aq) → Pbl2 (s) + 2KNO3 (aq)
Yellow precipitate of Pbl2 will be formed.
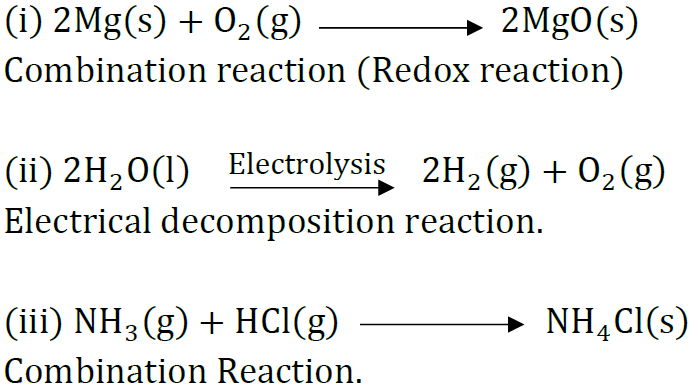
Question: State the type of chemical reactions and chemical equations that take place in the following:
(i) Magnesium wire is burnt in air.
(ii) Electric current is passed through water.
(iii) Ammonia and hydrogen chloride gases are mixed.
Answer:
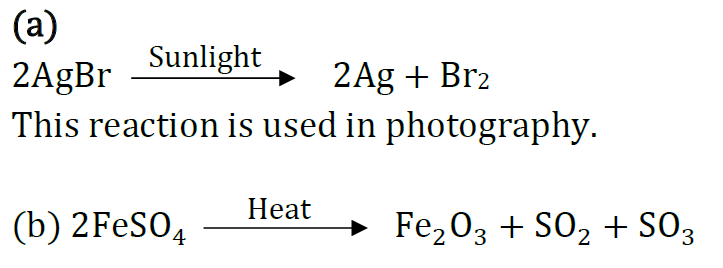
Question: (a) Write the essential condition for the following reaction to take place:
2AgBr → 2Ag + Br2
Write one application of this reaction.
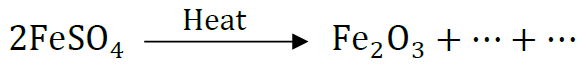
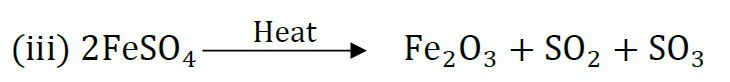
(b) Complete the following chemical equation of a chemical reaction 2FeSO4
(c) What happens when water is added to quick line. Write chemical equation.
Answer:
(c) Slaked lime is formed with hissing sound and lot of heat is evolved.
Question: 2g of ferrous sulphate crystals are heated in a dry boiling tube.
(i) List any two observations.
(ii) Name the type of chemical reaction taking place.
(iii) ‘Write the chemical equation for the reaction.
Answer: (i) (a) Green colour of FeSO4 disappears and reddish brown solid is formed.|
(b) Smell of burning sulphur.
(ii) Decomposition reaction
Question: Write chemical equation reactions taking place when carried out with the help of
(a) Iron reacts with steam
(b) Magnesium reacts with dil HCl
(c) Copper is heated in air.
Answer:
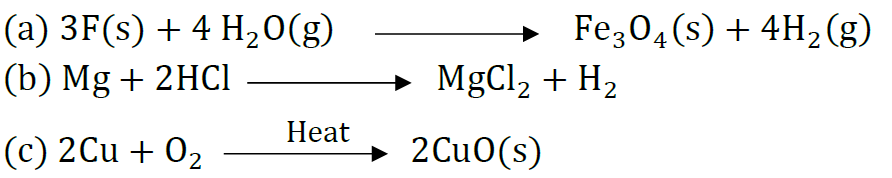
Question
If you collect silver coins and copper coins you may have seen that after some days a black coating forms on silver coins and a green coating on copper coins. Which chemical phenomenon is responsible for these coatings? Write the chemical name of the black and green coatings.
Answer:
Corrosion is responsible for the formation of this coating. Black coating is due to formation of Ag2S and green coating is due to formation of CuCO3.Cu(OH)2.
Question
When carbon dioxide is passed through lime water, it turns milky, why?
Answer:
Lime water (calcium hydroxide) combines with carbon dioxide to form a suspension of calcium carbonate which makes lime water milky.
Ca(OH)2 + CO2 → CaCO3 + H2O
Question
Identify the most reactive and least reactive metal: Al, K, Ca, Au.
Answer:
Most reactive metal: K(Potassium); least reactive metal: Au(gold).
Question
Take 3 g of barium hydroxide in a test tube, now add about 2 g of ammonium chloride and mix the contents with the help of a glass rod. Now touch the test tube from outside.
(i) What do you feel on touching the test tube?
(ii) State the inference about the type of reaction occurred.
(iii) Write the balanced chemical equation of the reaction involved. (Board Term I, 2017)
Answer:
(i) When barium hydroxide is added into ammonium chloride, the bottom of test tube is found to be cooler.
(ii) It is an endothermic reaction.
(iii) Ba(OH)2 + 2NH4Cl → BaCl2 + 2NH4OH
Question
(a) A solution of potassium chloride when mixed with silver nitrate solution, an insoluble white substance is formed. Write the chemical reaction involved and also mention the type of the chemical reaction.
(b) Ferrous sulphate when heated, decomposes with the evolution of a gas having a characteristic odour of burning sulphur. Write the chemical reaction involved and identify the type of reaction. (Board Term I, 2016)
Answer:
(a)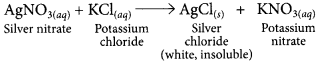
It is a double displacement reaction.
(b) Refer to answer 18(b) and (c).
Question
Name the type of chemical reaction represented by the following equation: (Board Term I, 2016)
(i) CaO + H2O → Ca(OH)2
(ii) 3BaCl2 + Al2(SO4)3 → 2AlCl3 + 3BaSO4![]()
Answer:
(i) Combination reaction.
(ii) Precipitation reaction or double displacement reaction.
(iii) Thermal decomposition reaction.
Question
What is a reduction reaction?
Identify the substances that are oxidised and the substances that are reduced in the following reactions. (Board Term I, 2015)
(a) Fe2O3 + 2Al → Al2O3 + 2Fe
(b) 2PbO + C → 2Pb + CO2
Answer:
Those reactions in which addition of hydrogen to a substance or removal of oxygen from a substance take place are called reduction reactions.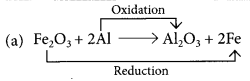
Fe2O3 is getting reduced to Fe and Al is getting oxidised to Al2O3.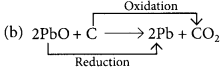
PbO is reduced to Pb and C is oxidised to CO2.
Question
(a) Can a displacement reaction be a redox reaction? Explain with the help of an example.
(b) Write the type of chemical reaction in the following:
(i) Reaction between an acid and a base
(ii) Rusting of iron. (Board Term I, 2017)
Answer:
(a) Consider the following displacement reaction:
Zn(s)+ CuSO4(aq) → ZnSO4(aq) + Cu(s)
Here, Zn has changed into ZnSO4 (i.e., Zn2+ ions) by loss of electrons. Hence, Zn has been oxidised. CuSO4 (i.e., Cu2+) has changed into Cu by gain of electrons. Hence, CuSO4 has been reduced. Thus, the above reaction is a displacement reaction as well as a redox reaction.
(b) (i) Neutralisation reaction
(ii) Oxidation reaction.
Question
Mention the type of chemical reaction that takes place when: (Board Term I, 2013)
(i) a magnesium ribbon is burnt in air.
(ii) limestone is heated.
(iii) silver bromide is exposed to sunlight.
(iv) electricity is passed through acidified water.
(v) ammonia and hydrogen chloride are mixed with each other.
Write the chemical equation for each reaction.
Answer:![]()
This is a combination reaction.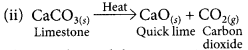
This is a thermal decomposition reaction.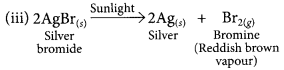
This is a photo decomposition reaction.![]()
This is electrolytic decomposition reaction.![]()
This is a combination reaction.
QuestionWrite chemical equation reactions taking place when carried out with the help of
(a) Iron reacts with steam
(b) Magnesium reacts with dil HCl
(c) Copper is heated in air.
Answer.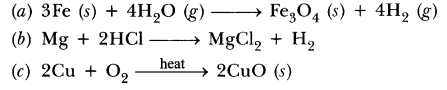
Long Answer Type Question [5 Marks] -Year 2014
Question(a) Write one example for each of decomposion reaction carried out with help of
(i) Electricity (ii) Heat (iii) Light
(b) Which of the following statements is correct and why copper can displace silver from silver nitrate and silver can displace copper from copper sulphate solution.
Answer.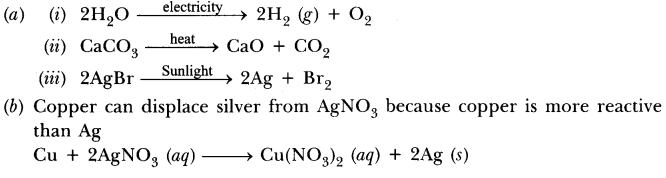
Short Answer Type Questions[ll] [3 Marks] -Year 2013
QuestionWhat is meant by skeltal type chemical equation? What does it represent? Using the equation for electrolytic decomposition of water, differentiate between a skeltal chemical equation and a balanced chemical equation.
Answer. The equations in which gaseous are written in atomic form instead of molecular form and equation is not balanced, are called skeltal type equation. They represent gaseous elements formed in atomic state and equation is not balanced
Short Answer Type Questions[l] [2 Marks]-Year 2012
Question.Identify the type of reaction(s) in the following equations.
(i)CH4 + 2O2 CO2 + 2 H2O
(ii) Pb(NO3)2 + 2KI ——–>Pbl2 + 2KNOs
(iii) CaO + H2O ——–> Ca(OH)2
(iv) CuSO4 + Zn ——–> ZnSO4 + Cu
Answer(i) Combustion reaction and oxidation reaction.
(ii)Double displacement reaction and precipitation reaction.
(iii) Combination reaction.
(iv) Displacement reaction.
QuestionWhat is the colour of ferrous sulphate crystals? How does this colour change after heating?
Answer.The colour of ferrous sulphate is pale green. The colour changes to reddish brown on heating due to formation of iron (III) oxide.
Give an example each for thermal decomposition and photochemical decomposition reactions. Write relevant balanced chemical equations also.
Thermal decomposition reaction: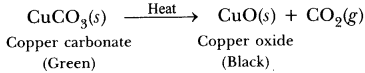
Photochemical decomposition reaction:![]()
QuestionWhy does the colour of copper sulphate solution change when an iron nail is dipped in it? Write two observations.
Answer. It is because a displacement reaction takes place.
Iron displaces copper from copper sulphate solution and forms pale green
coloured solution of FeS04 and reddish brown copper metal gets deposited.
Fe(s) + CuS04(aq) ——–> FeS04(aq) + Cu(s)
QuestionTranslate the following statement into a chemical equation and then balance it Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate. State the two types in which this reaction can be classified.
Answer. 3BaCl2(aq) + A12(S04)3(aq) ——–> 3BaS04(s) + 2AlCl3(aq)
It can be classified as double displacement as well as a precipitation reaction.
Question Why are decomposition reactions called the opposite of combination reactions? Write equations for these reactions.
Answer. In decomposition reaction, a compound is broken down into simpler compounds or elements, e.g.![]()
Combination reaction is a reaction in which two or more elements or compounds combine to form a new compound, e.g.![]()
Thus, decomposition and combination reactions are opposite to each other.
Short Answer Type Questions[ll] [3 Marks] -Year 2012
Question What is rancidity? Mention any two ways by which rancidity can be prevented.
Answer. The process in which taste and smell of food gets spoiled is called rancidity. It happens due to oxidation.
Prevention from rancidity:
(i) Antioxidants are added to fatty acids to prevent oxidation, e.g. chips are packed in presence of nitrogen gas which prevents spoilage by oxidation.
(ii)Food should be kept in airtight container in refrigerator.
Question Write balanced chemical equation for the reactions that take place during respiration. Identify the type of combination reaction that takes place during this process and justify the name. Give one more example of this type of reaction.
Answer. CgH1206 + 6O2 —————> 6CO2 + 6H20 + heat
It is an exothermic combination reaction because heat is evolved.
CH4(g) + 2O2(g) ————–>CO2 (g) + 2H20
The combustion of methane is another example of an exothermic combination reaction.
Question What is a redox reaction? Identify the substance oxidised and the substance reduced in the following reactions.
(i)2PbO + C —–> 2Pb + CO2
(ii)MnO2 + 4HCl —–> MnCl2 + 2H20 + Cl2
Answer. Those reactions in which oxidation and reduction take place simultaneously are called redox reactions.
(i) PbO is getting reduced and C is getting oxidised.
(ii) MnOs is getting reduced and HCl is getting oxidised.
Very Short Answer Type Questions [1 Mark] -Year 2011
Question State one basic difference between a physical change and a chemical change.
Answer. In a physical change, no new substance is formed, whereas, in a chemical change, new substance(s)/are formed.
Question: What is meant by the skeletal type chemical equation? What does it represent? Using the equation for electrolytic decomposition of water, differentiate between a skeletal chemical equation and a balanced chemical equation.
Answer: The equations in which gaseous are written in atomic form instead of molecular form and equation is not balanced, are called skeletal type equation. They represent gaseous elements formed in atomic state and the equation is not balanced.
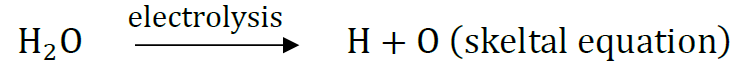
Hydrogen and oxygen are written in atomic forms and equation is not balanced.
H2O → H2 + O2 is also skeletal equation
2H2O → 2H2 + O2 is balanced equation
Question: What is rancidity? Mention any two ways by which rancidity can be prevented.
Answer: The process in which taste and smell of food gets spoiled is called rancidity. It happens due to oxidation. Prevention from rancidity:
(i) Antioxidants are added to fatty acids to prevent oxidation, e.g. chips are packed in presence of nitrogen gas which prevents spoilage by oxidation.
(ii) Food should be kept in airtight container in refrigerator.
Question: Write balanced chemical equation for the reactions that take place during respiration. Identify the type of combination reaction that takes place during this process and justify the name. Give one more example of this type of reaction.
Answer: C6H12O6 + 6O2 → 6CO2 + 6H2O + heat
It is an exothermic combination reaction because heat is evolved.
CH4(g) + 2O2(g) → CO2 (g) + 2H2O
Combustion of methane is another example of exothermic combination reaction.
Question: What is redox reaction? Identify the substance oxidised and the substance reduced in the following reactions.
(i) 2PbO + C → 2Pb + CO2
(ii) MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
Answer: Those reactions in which oxidation and reduction takes place simultaneously are called redox reactions.
(i) PbO is getting reduced and C is getting oxidised.
(ii) MnO2 is getting reduced and HCl is getting oxidised.
Question: Write the balanced chemical equations for the following reactions and identify the type of reaction in each case.
Thermite reaction, iron (III) oxide reacts with aluminium and gives molten iron and aluminium oxide.
Answer:
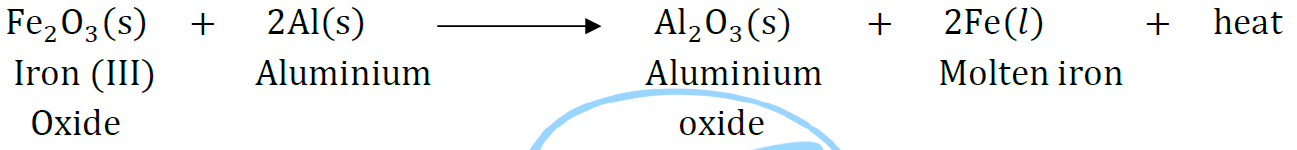
It is a displacement reaction because Al is displacing Fe from Fe2O3.
Molten iron is used for repairing broken railway tracks.
Question (a) Define a balanced chemical equation. Why should an equation be balanced?
(b) Write the balanced chemical equation for the following reaction:
(i) Phosphorus burns in presence of chlorine to form phosphorus penta chloride.
(ii) Burning of natural gas.
(iii) The process of respiration.
Answer: (a) Balanced chemical equation has an equal number of atoms of different elements in the reactants and products. According to law of conservation of mass, matter can neither be created nor be destroyed in a chemical reaction.
(b) (i) P4 (s) + 10Cl2 (g) → 4PCl5 (S)
(ii) CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O(l) + heat energy
(iii) C6H12O6 (s) + 6O2 (g) + 6H2O → 6CO2 (aq) + 12H2O (l) + energy
Question (a) Explain two ways by which food industries prevent rancidity.
(b) Discuss the importance of decomposition reaction in metal industry with three points.
Answer: (a) (i) Rancidity can be prevented by adding antioxidants to food containing fat and oil, e.g. butylated hydroxy anisole is added to butter as antioxidant.
(ii) It can be prevented by packaging fat and oil containing foods in nitrogen gas.
(b) (i) Molten NaCl is electrolytically decomposed to form sodium metal.
(ii) Aluminium metal is obtained by electric decomposition of bauxite ore mixed with cryolite.
(iii) Carbonate ores are thermally decomposed to give metal oxide which on reduction give metal.


